Cars get their energy from gasoline stored in engines.
Solar-powered households get their electrical energy from solar batteries packed with chemical fuel stores captured from sunlight.
But how is energy stored in your body for its later convenient use?
This is where cellular respiration comes in. Cellular respiration is a collection of reactions through which your cells can convert energy found in food into an energy-storing substance called ATP (Adenosine Triphosphate).
The process involves breaking a simple sugar molecule called glucose. This releases energy that goes into forming ATP.
However, for this to take place, an intricate process is followed (called metabolic pathways) and ATP is generated in successive stages in different quantities.
Let’s dig deeper.
3 Stage Process
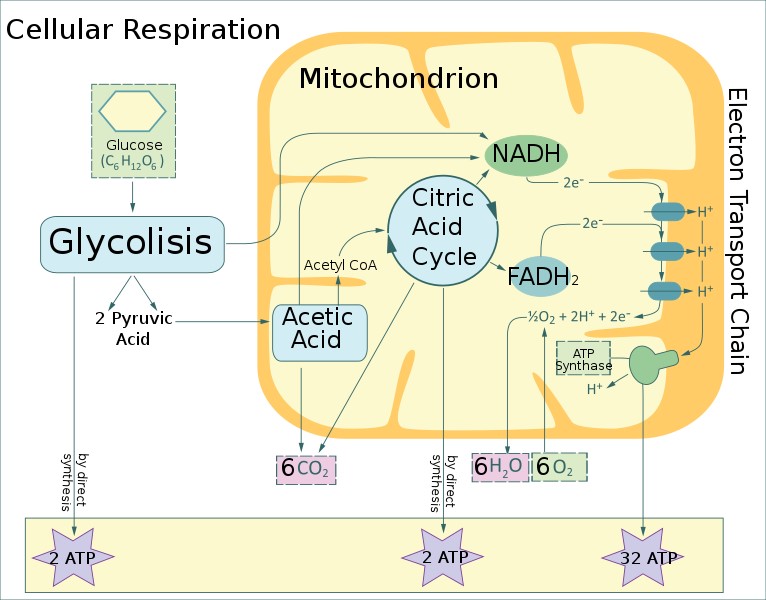
Stage One: (Glycolysis)
Almost all living things perform glycolysis (also called fermentation).
It is the first stage of the metabolic pathway in which ATP is produced by respiration.
It is also an anaerobic process because it occurs without oxygen.
During this process, glucose is broken down into a 3-carbon molecule called pyruvate.
Since energy is needed for this, two ATP molecules are used but at the end of glycolysis, 4 ATP molecules are produced to give us a net gain of 2 ATPs.
Glycolysis in summary:
- Inputs: Glucose + 2 ATPs
- Output: 2 Pyruvate molecules + 4 ATPs
- Location: takes place in the cytoplasm
Stage Two: (Kreb’s cycle, also called citric acid cycle)
In this stage, more ATP is produced.
Pyruvate arrives into the mitochondria for fuller oxidation (breakdown).
This forms more ATP along with NADH and FADH₂ (called carrier molecules).
Since 2 pyruvate molecules were received from the glycolysis, the Krebs cycle runs twice for each glucose that is used by glycolysis.
Two molecules, NAD+ and FADH, act as electron harvesters to prepare themselves for the next stage.
As a result, they are converted in the following way as they each pick electrons;
NAD+ → NADH
FADH → FADH₂
Oxygen is used and carbon dioxide is given out which is what you exhale when breathing.
Krebs cycle in summary:
- Inputs: 2 pyruvate molecules
- Output: 2 ATPS + CO₂ (waste) + FADH
- Location: takes place in mitochondria
Stage 3: (electron transport chain)
The two molecules NADH and FADH enter stage 3 loaded with electrons (thus carrying a negative charge).
They drop off these electrons which leads to a huge production of ATP.
Electron transport chain in summary;
- Inputs: NADH + FADH + Oxygen
- Output: 36 ATPs + H₂O (water)
- Location: takes place in the cristae (inner membrane of the mitochondria)
ATP Yield
As seen in the above process, one glucose molecule yields between 30 or 32 ATPs.
The stage with the lowest yield is glycolysis at only 2 ATPs.
The bulk of the ATPs produced occurs in the electron transport chain. This makes it the most efficient producer of ATP. This is because of its use of oxygen.
For these reasons, aerobic respiration produces larger quantities of energy, than anaerobic energy.
Respiration in Summary
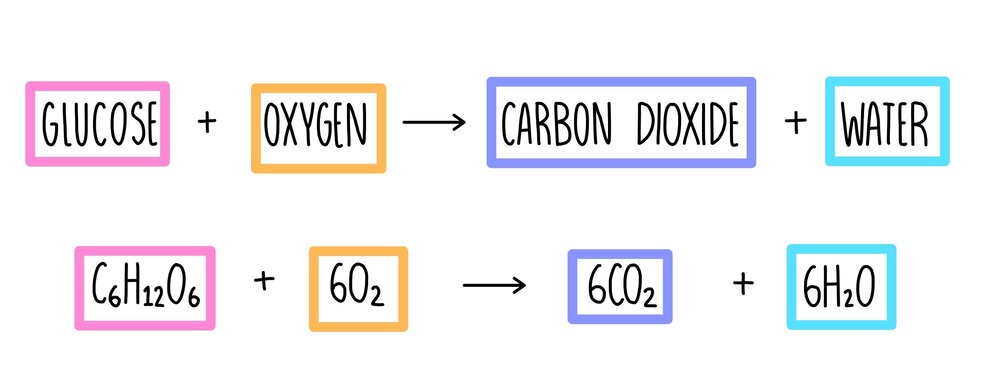
We can summarize the entire respiration process using the following simple reaction in terms of inputs and outputs.
Inputs Outputs
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + 28AT
Glucose + oxygen → carbon dioxide + water + ATP
After ATP is produced, it is stored as a short-term source of energy.
ATP enables energy to be released in smaller amounts, unlike glucose. It releases energy in exact amounts that are needed for specific tasks in cells.
We can compare the role of glucose and ATP to currencies. Glucose exists in bigger currencies or long-term storage currency.
ATP is like pocket change or smaller denominations. It can be used to power cellular processes requiring smaller amounts of energy.
Since ATP is not stored for long, it is produced for shorter times just before it is consumed again.
ATP and Energy Regulation
The amount of ATP varies at different times driven by the amount of energy available.
To get the energy from ATP, it’s broken down to ADP in the process below to release one of its phosphates;
ADP → ADP + Phosphate + Energy
The energy that is produced due to the phosphate released is used to perform the task the body needs to do.
When the cell breaks down food and has excess energy, it gets stored by reattaching the free phosphate back to ADP which makes it ATP again.
ADP + Phosphate + Energy → ATP
This back-and-forth conversion between ATP and ADP is a continuous cycle that regulates the amount of ATP and energy.
When there is excess energy, less ADP gets turned into ATP and when energy is needed, ATP breaks down to release the energy.
This action by ATP can be compared to a rechargeable battery. It can be reused several times efficiently like a rechargeable battery.
When it gives up its phosphate to become ADP, the ADP is not discarded. Instead, the ADP is like a run-down battery which can be recharged by reattaching a new phosphate to become ATP again.
Distribution of Mitochondria
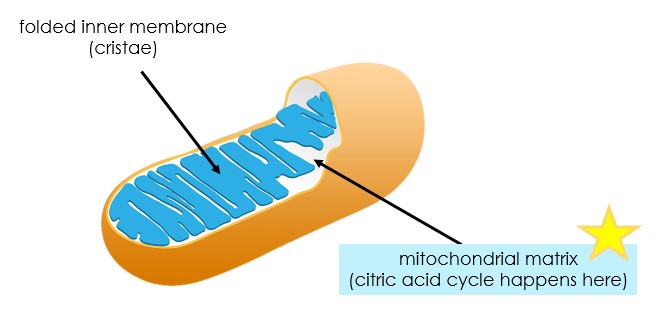
Mitochondria is the location where most ATP is created.
If a cell typically uses more energy, it will contain more mitochondria to meet its energy demands.
So, more ATP and energy will be stored there. For example, muscle cells are densely packed with mitochondria because of their unusually high need for energy.
Some organisms like bacteria do not have mitochondria despite their need for ATP. They do this through glycolysis. The process takes place in the cytoplasm.
How ATP Stores Energy
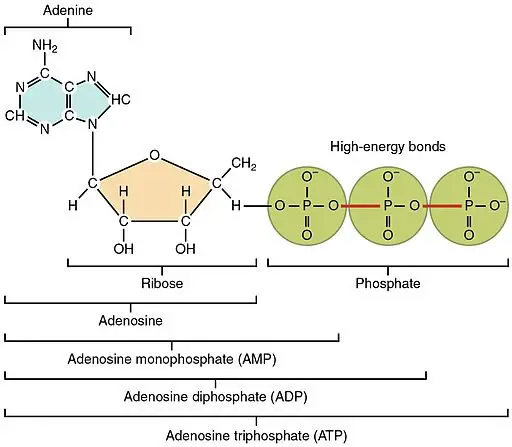
The key to ATP’s energy-storing capability is its structure.
It is a nucleotide made up of an adenine base with a ribose sugar connected to 3 phosphate groups.
It is the phosphate part that is responsible for ATP’s famous vast energy reserve.
These high-energy bonds called phosphoanhydride bonds bind the three phosphate groups.
Given that the three phosphate bonds highly repel each other, it takes massive energy to bind them which explains their high energy content.
Conclusion
Ever since life evolved reportedly some 3.5 billion years ago, ATP has faithfully served as power storage units for life’s processes.
From plants, bacteria to animals, this brilliant molecule executes its job to perfection in sustaining complex functions.
This universal use among all living things hints at a Grand Unity of Life that connects all life.
Image sources: 1, 2, 3, 4, 5