QUICK DEFINATION:
Weak nuclear forces are fundamental forces in particle physics responsible for certain types of radioactive decay and interactions between particles. They facilitate processes like beta decay and neutrino absorption, mediating interactions within atomic nuclei involving neutrinos and leptons.
BRIEF EXPLANATION:
Atoms comprise subatomic particles such as protons, electrons, neutrons, neutrino, and muon.
Electrons, photons, and muons are elementary particles.
Elementary particles are not composed of other particles. Protons, neutrons, and mesons are composite particles; they are composed of other particles.
Protons and neutrons, also known as nucleons, are found in the nucleus of an atom. They are made up of smaller particles known as quarks.
Quarks exist in flavors: up and down. A proton has two up quarks and one down quark, while a neutron has one up quark and two down quarks.
Atoms with a neutron-proton ratio of above 1.6:1 are unstable. This imbalance weakens the binding energy of the strong nuclear forces.
An unstable atom will try to attain stability by losing nucleons (protons and neutrons) or releasing energy in other forms.
When a neutron decays, a proton, electron, or neutrino is released. This type of reaction is called nuclear fission.
When light nuclei at a high velocity collide, they combine to form one heavy nucleus. This type of nuclear reaction is called nuclear fusion.
A typical example of nuclear fusion is the reaction between deuterium and tritium.
12H +33H → 24He +n +energy
Weak nuclear force initiates the reaction between deuterium and tritium, which supply energy in the sun.
The weak nuclear force is the force that causes radioactive decay in atoms. The force travels within a short distance—less than the length of a proton hence the name ‘weak interaction.’
EXAMPLES:
Here are examples of weak nuclear force in our daily life.
1. Fueling the sun

Heat energy in the sun is produced from the reaction between tritium and deuterium.
The heat from the sun sustains life. Without weak force, there won’t be enough heat from the sun to sustain life.
2. Radioactive decay
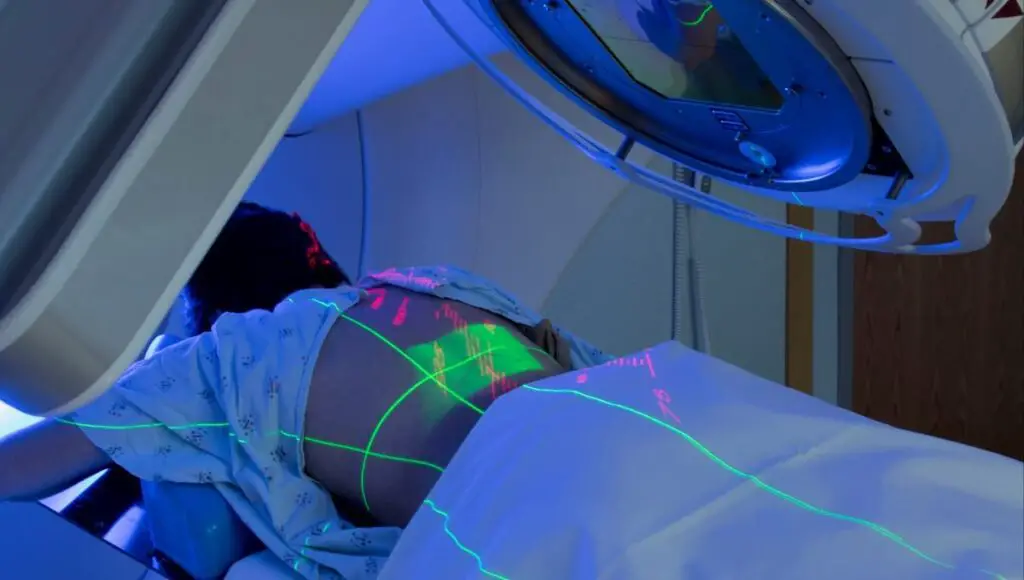
Weak forces cause radioactive decay. Radioactive decay is an essential reaction in the medical field in;
- Destruction of cancerous cells using the correct dose of radiation. Cobalt-60 and Caesium-137 are some of the radioisotopes used in cancer treatment.
- Treatment of patients with a defective thyroid
- Sterilization of surgical instruments
- Tracking of growth rate in bones and healing of fractures
3. Transmuting of particles
The weak nuclear force allows particles to transmute and form new particles. This reaction is essential in modifying particles to obtain the desired characteristics.
The muon is an example of a particle that transmutes to an electron and two neutrinos. The force also transmutes protons to neutrons and neutrinos.
4. Identifying quirks

It is possible to identify up and down quirks through their weak interactions. Identifying the quirks helps scientists in the analysis of the proton.
5. Determining the age of archeological materials

Archeologists can determine the age of fossils through carbon dating. Carbon -14 decays to nitrogen-14 through weak nuclear force. The amount of carbon -14 present in a fossil is used to estimate the age of the fossils.
6. Gauging the thickness of metal and paper sheets

The weak nuclear force causes radioactive decay. The particles emitted during radioactive decay are gamma, beta, and alpha particles.
The beta particles have high penetrating power and can pass through a thin sheet of paper or metal. This property of the beta particle helps gauge the thickness of metal and paper sheets.
7. Manufacture of atomic bombs and nuclear weapons

Atomic bombs and nuclear weapons are explosive devices. Nuclear weapons are made by combining chemical explosives with nuclear fission and fusion.
The explosives exert pressure on nuclear material, causing fission. When fission occurs, massive energy is released. This reaction creates a high temperature and pressure resulting in the ignition.
8. Gauging absorption of phosphate fertilizers

Phosphorus -32 is a radioactive isotope. It has more neutrons than protons (17). The most stable isotope of phosphorus has 16 neutrons while phosphorus -32 has 17. The high number of neutrons makes it unstable.
The absorption of phosphate fertilizers is gauged by analyzing phosphorus -32 in a soil sample. The amount of phosphorus-32 in the soil sample is compared to the amount of phosphate fertilizer applied. The amount absorbed is proportional to the amount detected in the soil sample.
Closing Thoughts
Among the four fundamental forces that keep the universe running, the weak nuclear force is the most significant.
David Armstrong, an American researcher, says that weak force controls life. Indeed it does. Light and heat from the sun are essential for life on earth. Humans, animals, and plants require sunlight to grow. Physical and chemical processes in our lives depend on energy from the sun.
Without weak force, the sun will lack energy, and life will freeze.


