Chemical energy is the potential energy stored in the bonds of atoms and molecules.
The potential energy is released once these bonds are broken. The energy can be released in the form of thermal energy or heat.
When you break the bonds, these atoms tend to move faster, colliding with each other in the process. This movement causes the temperature within the substance to increase and to be felt as heat.
Unfortunately, the transformation usually damages most substances as the bonds do not revert to their earlier states.
On the other hand, thermal energy is the energy that results from the movement of atoms and molecules in a substance. Heat is often released when the particles move fast.
Like other forms of energy, chemical energy can be converted to thermal energy. During such a conversion, energy stored in the bonds is released, causing the surrounding molecules to move faster. As a result, the thermal energy of the substance in question increases.
One question that most science students ask whenever they come across a scientific concept is whether the concept is applicable in day-to-day life. For instance, some students often wonder whether the conversation of chemical to thermal energy occurs in real life.
To keep you updated and help you appreciate science more, we’ll highlight for you 10 examples of chemical to thermal energy conversions in real life.
Let’s dive right in…
1. The Burning of Coal at Power Plants

Coal is a form of chemical energy stored between the atoms of the briquettes. During the burning process, these bonds break to generate thermal energy.
The chemical energy in the coal is effectively converted to heat for creating steam to run the turbines. Therefore, the thermal energy eventually becomes electricity. Coal is turned to ash in the conversion.
2. The Burning of Gasoline in a Car Engine
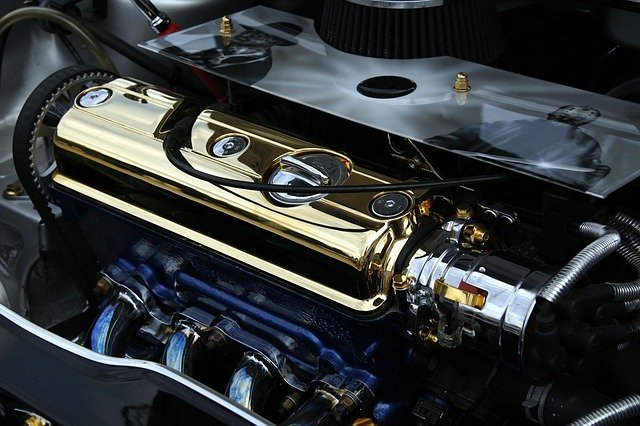
Car fuel is a form of chemical energy. When you start the vehicle, gasoline is injected into the combustion chamber. It burns within a closed compartment, causing small explosions.
These explosions cause the pistons in your car to move, eventually causing the car to move. They also cause the engine to heat up in the process.
Essentially, the chemical energy stored in gasoline becomes heat or thermal energy.
3. Natural Gas Burning in a Furnace
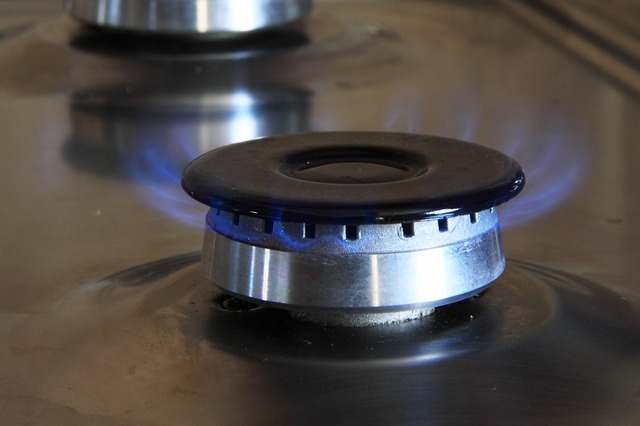
The natural gas you feed into a furnace or a gas cooker has chemical energy in its bonds. When it burns, this energy is converted to thermal energy.
The thermal energy heats the air around the furnace triggering a conventional current. This is the reason why you feel the heat when warming yourself via the vents from the furnace or standing next to your gas stove.
On the other hand, conduction causes the heat to transfer to the food in your cooking pot from the gas stove.
4. Hand Warmers

Hand warmers are common ways of keeping warm when outdoors, especially when hunting at night.
These warmers are usually made of different substances stored in a bag. You just mix the substances in the bag when you need to warm yourself.
These substances react with each other to produce heat. You feel warm by touching the bag after the reaction has taken place.
Once all the chemicals bonds are broken, the bag starts to cool down
5. Butane Torches

A butane torch burns a mixture of butane gas and oxygen to weld metal together. It generates heat that is hot enough to melt some types of metal.
Oxygen is used to help burn butane gas efficiently. During the process, the chemical bonds in the gas are broken to release thermal energy.
The burning process also causes the gas to emit a blue light that can be increased and reduced by adjusting the oxygen levels.
6. The Sun

The sun is made is made of hot plasma that converts hydrogen gas into helium. The process results in intense heat that is felt across the solar system.
Hydrogen atoms are caused to collide with each other through various reactions, thereby losing the energy stored in their bonds.
Eventually, the reaction forms the inert helium gas.
7. Geothermal Energy

Geothermal energy is heat produced from the earth’s crust. This heat is usually produced by the hot magma, which is made of molten rock.
Much of the geothermal energy is caused by the radioactive decay of materials under the earth’s crust.
Radioactive decay is a chemical reaction that breaks down material over time. The high pressure in the earth’s crust increases the heat.
This heat burns hot water beneath the earth’s surface, which, in turn, drives the turbines on the surface.
8. Lighting a Candle

Candles produce light through a chemical reaction between the wax, wick, and oxygen.
The wick helps facilitate the reaction between wax and oxygen, which produces a blue light where there is enough oxygen and bright yellow where there is little oxygen. In the process, heat is also produced.
Unlike many materials, you can reuse the wax in a similar energy conversion process.
9. Magnesium Lighters

Magnesium lighters create a fire when magnesium and oxygen combine in a chemical reaction. This causes magnesium to burn, forming a white powder called magnesium oxide.
The reaction creates thermal energy and a spark that triggers fire.
10. Nuclear Reactors

Nuclear reactors work by splitting the bonds between atoms in radioactive material. This process is called fission.
The splitting of chemical bonds releases kinetic energy and gamma radiation, which causes the substances to heat.
The thermal energy heats the water, which turns the turbines to produce electricity.
Closing Thoughts
There you have it; quick conversion of chemical energy to thermal energy examples in real life.
Anything that is burned to produce heat converts chemical energy to thermal energy. This includes the burning of waste and papers or mixing chemicals that produce heat in the lab.
Check around your home for other examples of chemical to thermal heat conversion.


