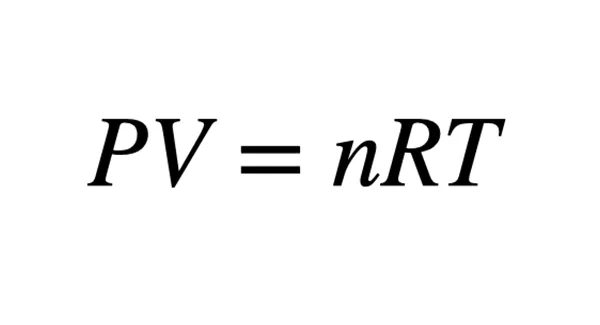Gas behavior is very abstract since it involves countless atoms interacting with each other and their environment. However, scientists have been able to describe the properties of gases by creating laws such as the ideal gas law.
The ideal gas law is a mathematical formula that relates pressure, volume, temperature, and the number of particles of a gas. It demonstrates the relationship between gas properties, allowing scientists to predict gas behavior.
The ideal gas law equation is PV = nRT where P is the gas pressure in Pascals, V is the volume of the gas in meters cubed, n is the number of particles in moles, R is the gas constant, and T is the temperature in Kelvin.
While the ideal gas law is purely theoretical, we can observe its effects in many real-life situations. Here are 11 examples of the ideal gas law in action.
Examples of Ideal Gas Law in Our Daily Life
1. Balloons
When we inflate a balloon by blowing air into it, we create a gas container. The amount of air we put inside determines the pressure within the balloon. As more air is added, the pressure increases proportionally. However, if too much air is forced into the balloon, the pressure will eventually reach a point where it becomes too strong for the balloon to contain. This excessive pressure causes the balloon to burst or open as it can no longer withstand the force exerted.
2. Car tires
The ideal gas law provides us with the necessary information to calculate the precise amount of air pressure required for a vehicle’s tires. Fluctuations in temperature can impact the quantity of pressure needed. Therefore, it is highly recommended to monitor your tire pressure regularly and fill it up as necessary to maintain optimum levels for safe driving. By doing so, you can guarantee that your car performs optimally and remains secure on the road, avoiding potential hazards and accidents.
3. Baking
Baking requires accuracy in measuring temperature and volume. To ensure that the food being baked turns out perfectly, bakers need to know how much gas will expand or contract during the baking process. This is where the ideal gas law comes in handy. The ideal gas law helps bakers calculate how much gas will be produced when they cook their food at specific temperatures and volumes.
4. Air conditioners
The refrigerant in an air conditioning system goes through a cycle of being compressed and then expanded again. This process changes its temperature and allows it to cool down the air inside your home. When you turn on your air conditioner, it compresses a particular type of gas called refrigerant, which makes it very hot. Then this hot gas moves through some coils where it cools down and turns into a liquid. After that, the liquid is allowed to expand back into a gas again, which causes it to become frigid. Finally, this cold gas moves through another set of coils where it absorbs heat from inside your home before being compressed once more and starting the whole cycle over again.
5. Scuba Diving
Scuba divers need to breathe air, but since there is no air underwater, they use tanks filled with compressed air that they carry on their backs. The amount of air a diver needs depends on how deep they go and how long they stay underwater. The ideal gas law helps scientists predict the pressure, volume, and temperature of gases (like the compressed air in a tank) at different depths.
Also check: 10 Real Life Examples of Combined Gas Law
6. Gas tanks
The ideal gas law is crucial in developing automobile gas storage tanks. The temperature and pressure inside it directly influence the capacity of a gas tank, necessitating designers to meticulously consider factors such as the tank’s size and the environment in which it will be used. As a result, they must ensure that every component is perfectly aligned to achieve optimal performance and maximum efficiency.
7. Weather
The atmosphere is like a big gas that surrounds the Earth. Meteorologists use the ideal gas law to help them figure out what’s going on in the atmosphere. This law allows them to calculate things like temperature (how hot or cold it is), how much force there is pushing down on something, and composition (what gases are in the air). By using this information, meteorologists can make predictions about what kind of weather we can expect.
8. Airbags
Airbags inflate rapidly upon a collision by relying on the ideal gas law. When a car crashes into something at high speed, it generates an enormous amount of force that causes sensors in the car’s body to trigger an electrical signal that inflates the airbag. The airbag contains a unique mixture of chemicals that react when exposed to heat or other forms of energy. As these chemicals react, they produce large amounts of gas, which fills up the bag very quickly and creates enough pressure inside the bag to cushion anyone who might be sitting nearby during an accident.
9. Rocket engines
The ideal gas law is used in the design of rocket engines to figure out how much pressure and heat are needed for fuel combustion and propulsion. Burning fuel creates hot gases that exit the engine at high speeds. This action produces thrust, which propels the rocket forward. The law allows experts to calculate precisely how much pressure and temperature are required to create this kind of reaction and thus design more efficient rocket engines with better performance capabilities.
10. Steam engines
Steam engines were one of the first machines to use the ideal gas law. In a steam engine, the expanding steam was used to push pistons back and forth, which powered other machinery. This allowed factories and other industries to operate more efficiently because they could rely on these powerful machines instead of relying solely on human or animal labor.
11. Welding
Welding involves using gases for heating or shielding, such as oxy-fuel cutting and arc welding. The ideal gas law helps welders determine the amount of gas required for a particular task and how to regulate its flow rate effectively. It enables welders to calculate the necessary pressure, volume, and temperature of the gas needed to achieve optimal welding results.