We depend on electricity to carry out various tasks.
Electric energy provides us with light to see in the dark. It powers our smartphones, televisions, and computers. It even powers our trains and vehicles. Put simply, electrical energy is the driving force of our economy and our modern lives.
While electrical energy today can be produced in different ways, it was first observed as a result of chemical reactions that later led to the development of chemical cells.
There are now many more examples of chemical energy being converted to electrical energy.
Let’s explore a few conversions of chemical energy to electrical energy examples:
1. Chemical Cells
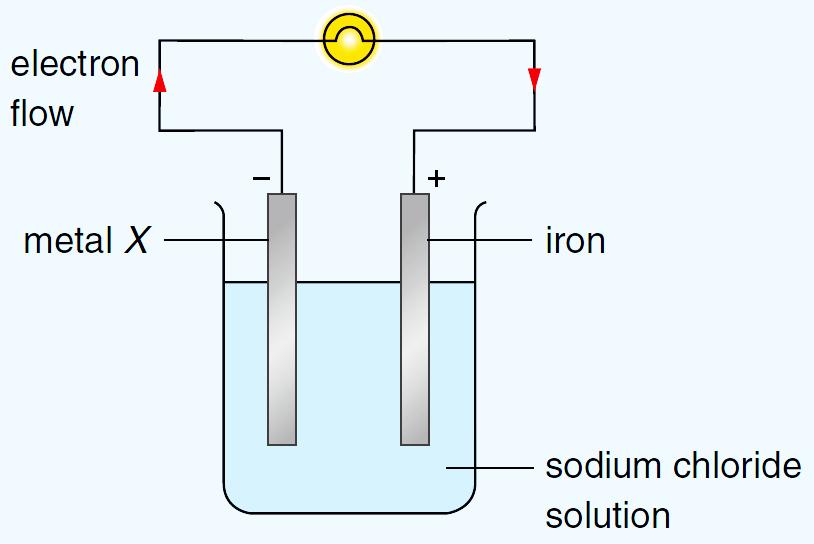
Chemical cells are devices that produce electrical energy from chemical energy.
Chemical cells consist of two electrodes placed in alkaline or acid solution.
The first cells invented used copper and zinc electrodes in an alkaline solution. Two or more cells are combined to form a battery.
Batteries are used to provide electrical energy to power various devices today ranging from our flashlights and remote controls to vehicles and laptops.
2. Fuel Cells
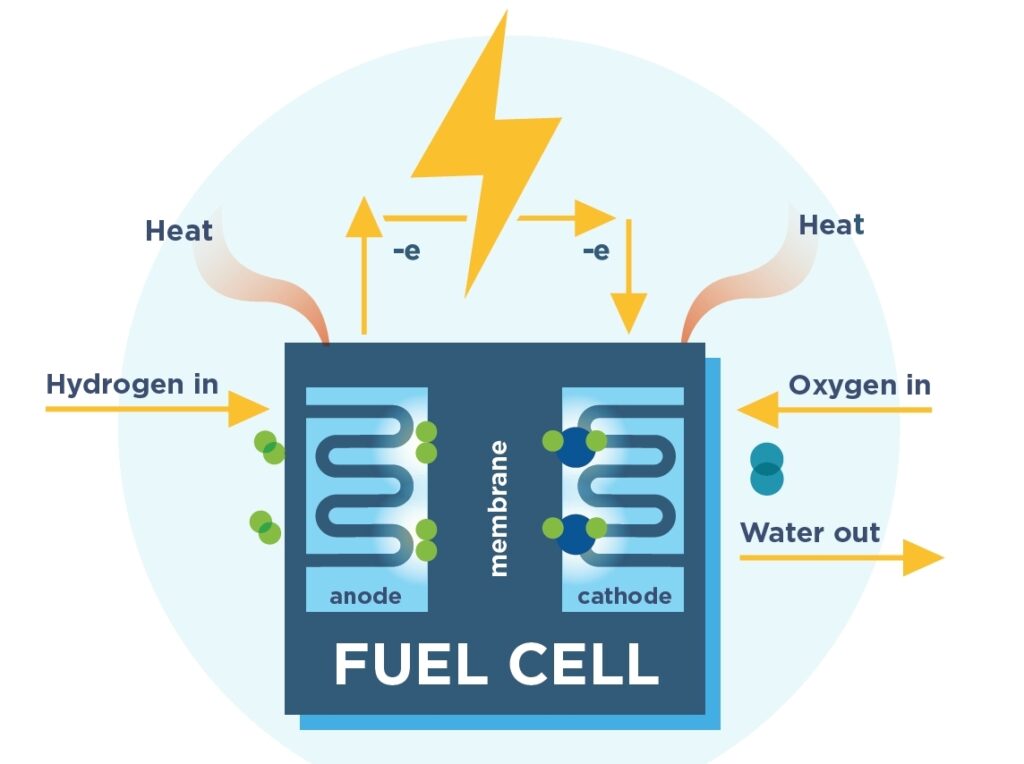
Fuel cells convert chemical energy into electrical energy.
Fuel cells use an oxidizing agent such as oxygen to oxidize hydrogen in a pair of redox reactions. An electric current is formed as a result of the reaction.
These cells can produce electric energy continuously as long as the supply of hydrogen and the oxidizing agent are constant.
Fuel cells are considered a better source of energy compared to combustion as the only byproducts of the reaction are water and oxygen.
Fuel cells have found a wide range of applications in transportation, as emergency backup power and for portable sources of energy.
For example, fuel cells are used to power NASA’s space crafts as they not only provide energy continuously but also drinking water for the crew.
3. The Nervous System
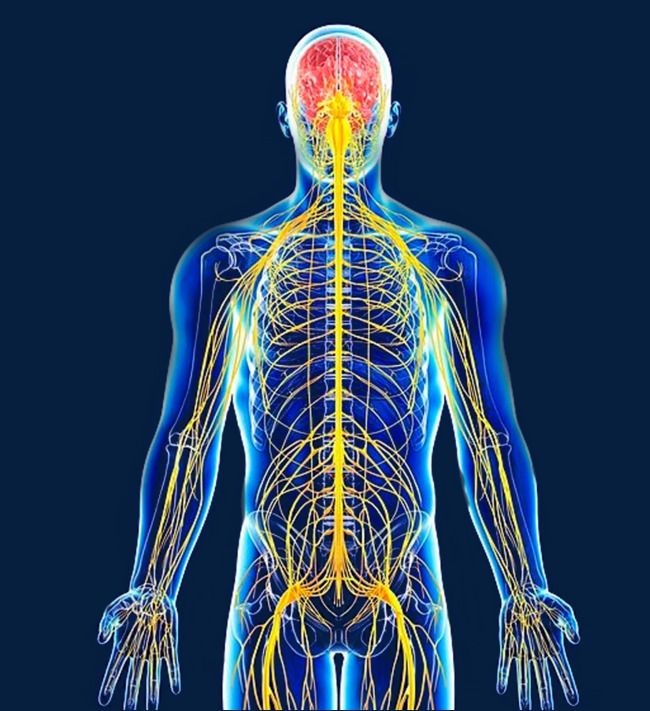
The nervous system controls the functions of the body. It relies on the transmission of messages from different parts of the body to the brain and from the brain to other parts of the body.
The nervous system relies on electricity to send signals throughout the body. This is made possible through the use of charged elements like calcium, magnesium, potassium and sodium to generate electricity.
If the electrical currents are disrupted, an illness may develop. The electric currents can also be witnessed in other cells throughout the body.
4. Potato Batteries
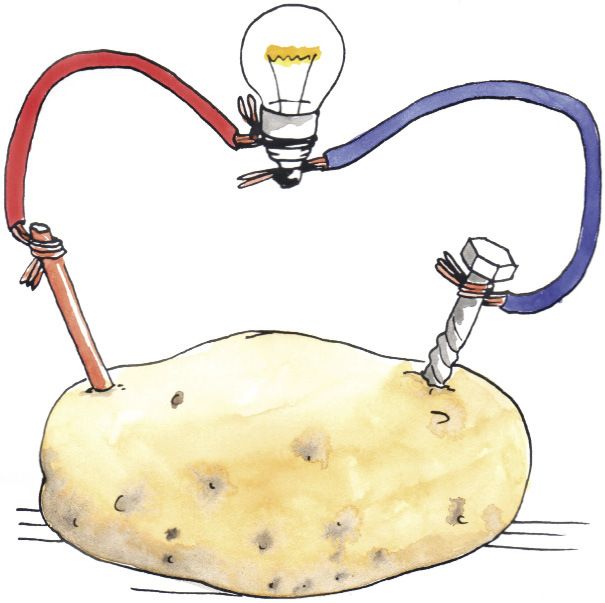
Potatoes are great for French fries. They are also great for producing electrical energy.
Potato batteries can be made by inserting two electrodes (commonly zinc and copper) into a potato.
The acid within the potato undergoes a chemical reaction, which results in the production of electrical energy.
Several potatoes can be combined to produce even more energy.
5. Mercury Cell

Mercury cells are non-rechargeable batteries. They produce electrical energy using a reaction between zinc electrodes and mercuric oxide in an alkaline electrolyte.
Mercuric oxide or mercury (II) oxide is a non-conductor. It is mixed with graphite to form the cathode.
Zinc is used for the anode in the cell. A porous material is soaked with electrolyte to form the salt bridge.
Zinc is oxidized to form zinc oxide and mercuric oxide is reduced to form elemental mercury.
6. Lithium-Ion Batteries

These batteries function much of the same way as chemical cells. They have an anode that stores lithium made from carbon, a cathode that stores lithium made from metal oxide, and an electrolyte that carries positively charged lithium ions.
When the battery is producing electrical energy, the positive ions move from the anode to the cathode. A separator that is included in the cell contains the electrolyte that promotes this movement.
However, unlike chemical cells, the movement of ions can be reversed. This ability makes lithium-ion batteries rechargeable.
These batteries feature in smartphones and laptops.
7. Electric Eels

Electric eels produce electric energy as a defense mechanism as well as for hunting prey.
The electricity is produced by special electric organs i.e. Hunter’s organ, Sachs’ organ, and the main organ. These organs are located in the abdominal and tail region of the eel’s body.
The organs contain metal ions that are dissolved in fluid in and around the cells within the organs.
Each cell in these organs carries a small negative charge. The cells are activated by signals from the eel’s nervous system.
Each cell acts like a battery generating electrical energy.
8. Electric Sting Rays

There are over 69 species of stingrays that produce electricity. These stingrays use electrical energy to stun prey. Others use it as a defense mechanism while many others use it for navigating the open seas.
The electrical energy is generated in cells that are known as electrocytes. When these cells are stimulated, ions move across the cell membrane. This results in an electric discharge.
There you have it; a few conversions of chemical energy to electrical energy examples. Generally, electrical energy can be generated in various ways. Many of these ways involve converting chemical energy into electrical energy.
