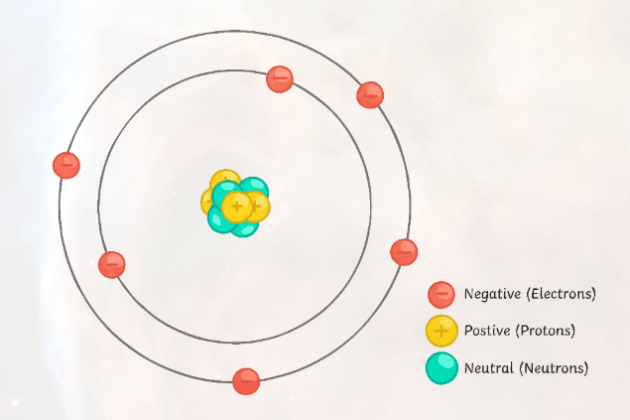
(a) neutrons
(b) electrons
(c) protons
(d) neutrons and protons
(e) protons, neutrons, and electrons.
The Correct answer is (c) Protons
Explanation
Atoms of the same element have the same number of protons in their nucleus.
Let’s break it down…
The number of protons in an atom is also called its atomic number. Now, atomic number is very important to chemists and scientists because it is unique for atoms of an element.
For instance, you can distinguish between different elements in the periodic table by knowing their atomic (or counting the number of protons in their nucleus).
An atom with 1 proton is always hydrogen; with two protons is Helium; and with three protons is Lithium; with 4 protons is Beryllium; 6 protons is carbon, etc.
To this end, we hope that you can easily see that every element has different atomic number (or different number of protons in its atom).
If the atomic number (number of protons) is changed, the element is changed.
Atoms of the same element, therefore, must have the same number of protons.
But atoms of the same element can have different numbers of neutrons.
For instance, all hydrogen atoms have one proton but may contain different numbers of neutrons.
Such atoms—belonging to the same element but with different numbers of neutrons—are often referred to as isotopes.
Let’s now explore electrons…
A neutral atom must have one electron for every proton. So, if you know the atomic number of an element, you can easily tell the number of electrons in a neutral atom of that element.
For ions (positively or negatively charged atoms), the number of electrons changes.
For instance, if the ion is positively charged, it means that an electron has been removed from its configuration and if it is negatively charged, then an electron has been added to the configuration.
Here is how a neutral and a charged chlorine atom look like:
Neutral Cl atom (atomic number = 17): 1s2 2s2 2p6 3s2 3p5
Charged Cl-1 atom (atomic number = 17): 1s2 2s2 2p6 3s2 3p6 (gain 1 e−)
As you can see, the atomic number of chlorine doesn’t change (whether it is neutral state or ionic state).
The number of protons remains the same regardless—which confirms our answer or first statement: Atoms of the same element have the same number of protons in their nucleus.
Learn more here: Labeled diagram of an atom (Including History and Important Properties)