a. γ−rays
b. visible light
c. ultraviolet radiation
d. infrared radiation
e. visible red light
f. visible blue light
g. All of these have the same energy.
Answer:
The answer is (a) γ−rays (gamma rays)
Explanation:
Radiation is a form of energy that is transmitted through space or other media as waves.
For light, this is known also as electromagnetic radiation or EMR. It is energy that carries both magnetic and electric fields.
The wave action is due to the vibrating effect on electrical charges caused by the electric and magnetic fields.
Source: NASA via wikimedia
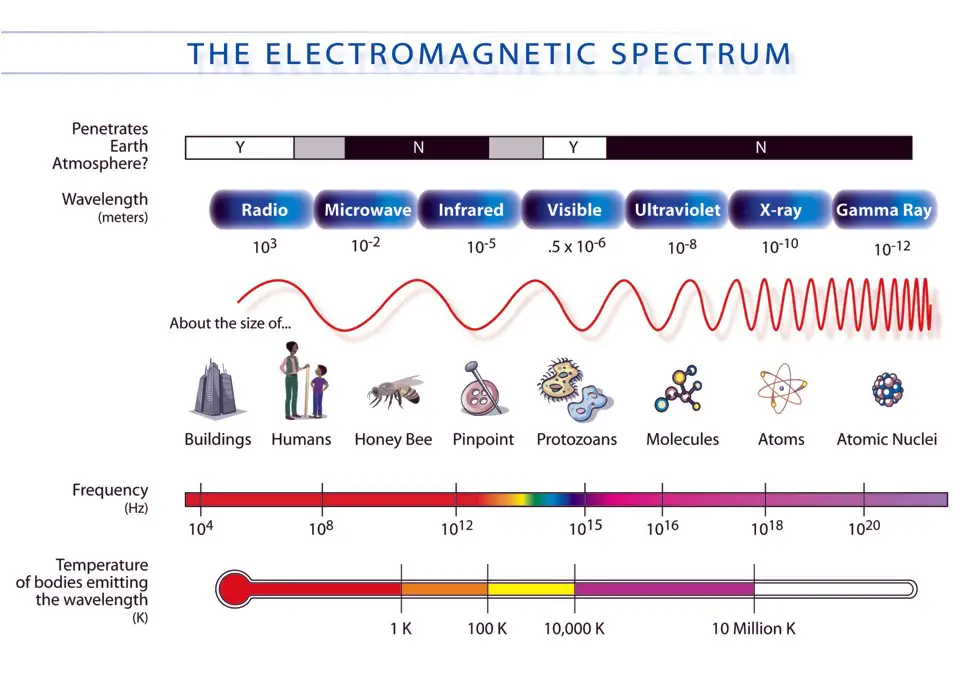
EMR is made up of different waves of varying wavelengths. They can be represented in an EMR spectrum that looks like a rainbow.
Each type of wave is found in a particular part of the EMR spectrum. For example, radio waves are found at one end of the spectrum and gamma rays at the end of the spectrum. All the rest lie in between.
Each wave can be differentiated from the other by its wavelength and frequency.
There are 7 types of waves in total found in the EMR spectrum:
- Gamma rays
- Radio waves
- Visible light
- Ultraviolet radiation
- X-rays
- Infrared radiation
- Visible red light and
- Visible blue light.
Except for visible light, you can’t see any of the other EM waves.
Wavelength, Frequency, and Wave Formula
The wavelength and frequency of a wave have an inverse relationship. This means when the frequency is high, the wavelength is small and vice versa.
We can write the formula of any wave as:
ν= λf
Where:
ν = velocity of the wave
f = frequency (number of waves/cycles per second)
λ=wavelength (distance separating two identical points on the wave, measured in nanometers or nm)
For EM, the value of ν is the speed of light in a vacuum which is c. So, we can write the equation as:
c =λf
c is a constant which is 3.0×10⁸ m/s.
Wave Energy
Each type of wave has a different amount of energy.
As we saw in the equation, the shorter the wave, the higher the frequency.
Also, the higher the frequency, the higher the energy the wave has.
The energy produced by EM waves can be calculated using the formula:
E= hc/λ
Where:
E = energy (in joules)
h= Planck’s constant (6.62607004 × 10-34 m2 kg / s)
c=speed of light (3.0×10⁸ m/s.)
λ= wavelength

From the formula, we can see that the lower the wavelength (i.e., the higher the frequency), the higher the energy.
Since gamma rays have the shortest wavelength, it also has the highest frequency and ENERGY than all the other waves.
Because of their high energy, gamma rays can be destructive to human tissue.
The wave with the weakest energy is radio waves since it has the longest wavelength and therefore the lowest frequency.

