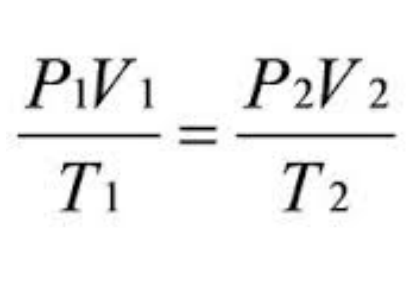The combined gas law describes the relationship between the pressure, volume, and temperature of a fixed amount of gas.
This law is a combination of three gas laws: Boyle’s law, Charles’ law, and Gay-Lussac’s law.
The combined gas law is often used in thermodynamics to analyze the behavior of gases in various conditions, such as during chemical reactions or in gas compressors.
The formula for the combined gas law is:
P1 * V1 / T1 = P2 * V2 / T2
Where:
P1 = initial pressure
V1 = initial volume
T1 = initial temperature
P2 = final pressure
V2 = final volume
T2 = final temperature
To use this formula, the number of moles of gas is kept constant. This means that the amount of gas in the system should remain fixed throughout the experiment.
The combined gas law is a fundamental concept in the world of chemistry, and its application can be found in a wide variety of real-life situations.
Let’s explore 10 real-life examples of the combined gas law in action.
1. Automobiles
When you drive a car, the engine uses a mixture of air and fuel to create energy that moves the car forward. But before the fuel can ignite and create this energy, it needs to be compressed into a smaller space. This happens inside the cylinder of the engine.
As the air-fuel mixture gets compressed, its pressure increases while its volume decreases. This creates a lot of force—enough to move your entire car. When the spark plug ignites the fuel, it releases even more energy which propels your car forward.
2. Scuba Diving
When scuba divers go deeper into the water, they experience increased pressure due to the weight of all the water above them. The increase in pressure causes the air that they are breathing to become compressed and take up less space than it did at the surface level. This volume change can affect how much air a diver has left in their tank and how long they can stay underwater before needing to resurface for more air. The combined gas law is used by scuba divers and helps them understand these changes in pressure and volume so that they can safely explore underwater environments without running out of air or experiencing other dangerous effects from being deep underwater.
3. Climate Control Systems
When it comes to climate control systems, compressing and expanding the refrigerant gas is what allows for cooling or heating. When the gas is compressed by the system’s compressor unit, it gets hotter because there’s less space for it to occupy. Then when that compressed gas expands again into a larger area (like inside your house), it cools down because there’s more room for it to spread out.
4. Cooking
When you apply heat to your food, the gas inside it (like water or air) starts to expand. As a result of this expansion, the volume of your food increases too. This is why rice or pasta swells up when it’s cooked. The same thing happens with bread dough too. As it bakes in the oven and gets hot, the gases trapped inside cause it to rise and become fluffy. Essentially, cooking involves more than just adding ingredients together; there are all sorts of chemical reactions happening behind the scenes.
5. Hot Air Balloons
Hot air balloons work by using the combined gas law. When you heat a gas (like air), it expands and takes up more space. When this happens inside a hot air balloon, the volume of gas inside the balloon increases and makes the balloon rise into the sky.
When there’s more hot air inside the balloon, it becomes less dense than the cooler air outside of it. So, as long as there’s enough heat to keep making more hot air in the balloon, it will continue to rise higher and higher.
Also Check: 11 Real-Life Examples of Ideal Gas Law
6. Tire Pressure
Have you ever wondered why it’s important to check your car tire pressure? Well, the pressure inside your tires is an example of the combined gas law. This means that as the air in your tires heats up, it expands and causes the pressure inside to increase.
So, if you don’t regularly check your tire pressure and adjust it when needed, then you could be driving on underinflated or overinflated tires. This can lead to poor fuel efficiency, uneven tire wear, and even unsafe driving conditions.
7. Air Conditioning
Air conditioning systems work by manipulating the pressure, volume, and temperature of air. The refrigerant inside the air conditioner is compressed, which increases its pressure and temperature. Next, it passes through a condenser coil where it releases heat and becomes a high-pressure liquid. This liquid then passes through an expansion valve where it expands rapidly, resulting in a decrease in pressure and temperature. Finally, the cold refrigerant flows through an evaporator coil where it absorbs heat from the surrounding air and cools it down. This process continues until the desired temperature is achieved.
8. Weather Patterns
The Combined Gas Law can also help explain weather patterns on Earth. The law says that changes in pressure, temperature, and volume of gases are all related to each other. When it comes to weather, changes in atmospheric pressure (the weight of the air around us) can cause shifts in wind patterns which can lead to things like hurricanes or tornadoes. Temperature changes also play a role because they affect how dense the air is. Dense air means higher atmospheric pressure while less dense air means lower atmospheric pressure. So when there are differences in temperature across an area, it can create areas of high and low pressure which then affect wind patterns and ultimately lead to different types of weather conditions.
9. Chemical Processing
Chemists use the combined gas law to help them optimize reactions during chemical processing. The law helps chemists control pressure and temperature, which ensures that a reaction happens in the best way possible. By controlling these factors, they can make sure that their reaction goes smoothly and produces the desired results. This is important because if too much or too little pressure or temperature is applied, it can cause problems with the reaction and lead to undesirable outcomes.
10. Breathing
Breathing is something we do all the time without even thinking about it. But did you know that there’s a scientific explanation for how it works? When we breathe in, our lungs expand and the pressure inside them decreases. This decrease in pressure causes air from outside to rush into our lungs because there is more atmospheric pressure outside than inside.
Think of it like sucking on a straw. When you suck on a straw, you create a vacuum inside your mouth which causes liquid to be drawn up the straw and into your mouth. Similarly, when we inhale, we create a vacuum in our lungs which draws air into them.