Tonicity refers to the concentration of solutes on either side of a semipermeable membrane that separates two solutions. It is a fundamental concept in biology used in various physiological processes like osmoregulation and cell communication.
What are the different types of toxicity?
Types of Tonicity
There are three types of tonicity based on the concentration of solutes:
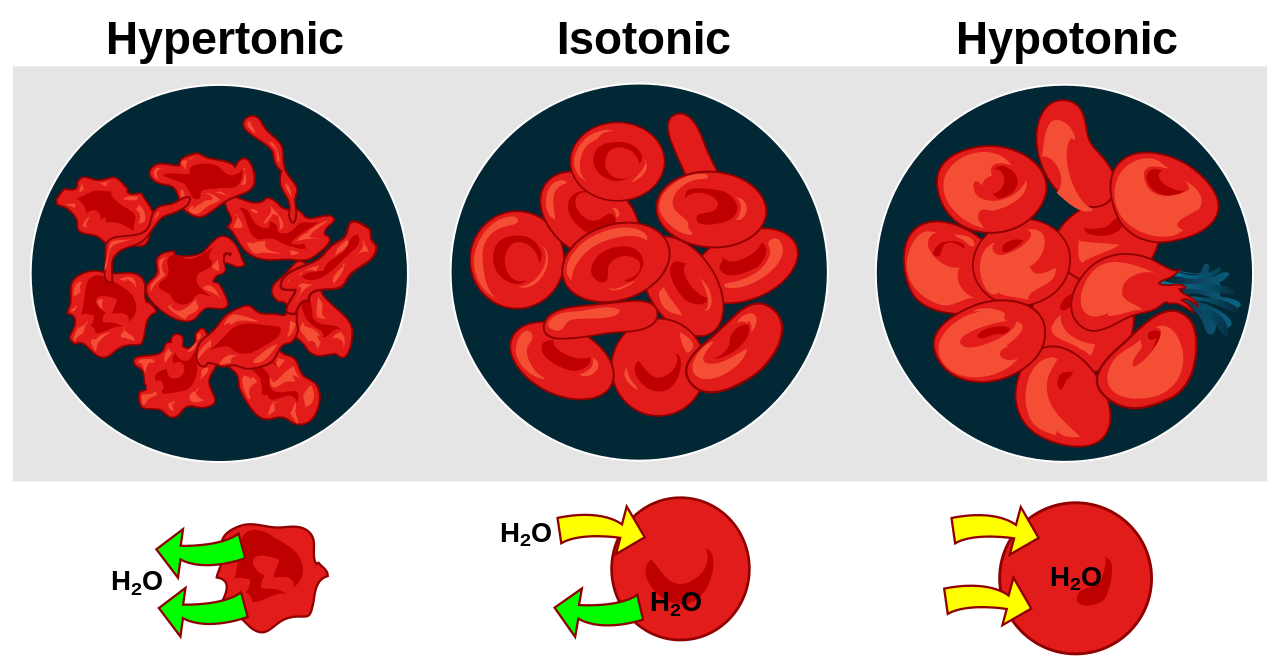
A. Hypertonic Solution
A hypertonic solution has a higher concentration of solutes compared to another solution. Water molecules move from the side of lower solute concentration to the side of higher solute concentration when two solutions of different tonicity are separated by a semipermeable membrane, resulting in the shrinkage of the cells in the solution with higher tonicity.
B. Hypotonic Solution
A hypotonic solution has a lower concentration of solutes compared to another solution. Water molecules move from the side of higher solute concentration to the side of lower solute concentration when two solutions of different tonicity are separated by a semipermeable membrane, causing the cells in the solution with lower tonicity to swell and burst.
C. Isotonic Solution
An isotonic solution has an equal concentration of solutes as another solution. When two solutions of equal tonicity are separated by a semipermeable membrane, there is no net movement of water molecules across the membrane, and the cell in the solution stays the same.
Application of Tonicity
Tonicity is essential in various biological processes, such as regulating blood pressure, maintaining electrolyte balance, and regulating cell volume. The difference in tonicity between the intracellular and extracellular environments determines the movement of water and ions across the cell membrane, which is necessary for various cellular functions. Disruption of tonicity can lead to several pathological conditions, such as dehydration, edema, and cell damage.
Tonicity Examples in Real Life
The following are tonicity examples in real life.
1. Sea Creatures
Marine animals, like fish and whales, have come up with different ways to deal with the saltiness of the water they live in. They’ve developed osmoregulatory systems that help them maintain the right balance of salt and water in their bodies. This is important because if there’s too much or too little salt in their bodies, it can cause problems like dehydration or swelling.
2. When We Cry
Tears are a hypotonic solution, meaning they have a lower concentration of solutes than the fluids in our eyes. When we cry, tears flow out of our eyes and mix with the fluids on the surface of our eyes. The difference in tonicity between the two solutions causes water molecules to move from the side of higher solute concentration (the fluids in our eyes) to the side of lower solute concentration (our tears), which helps flush out irritants and keep our eyes moist.
3. Sports Drinks
Sports drinks have the same amount of dissolved substances as our body fluids. This makes them easy for our bodies to absorb quickly and helps replace electrolytes that we lose when we sweat during exercise. Electrolytes are essential minerals in our bodies that help control muscle function and hydration levels. So, sports drinks can be helpful for athletes who need to stay hydrated and keep their muscles working well during intense workouts or games.
4. IV Fluids
In hospitals, when patients require fluids to be administered directly into their veins, the goal is to align the balance of chemicals and water with that found in our blood. This is important because if the fluid going into our veins does not have this same balance, it could hurt our cells or change how much different chemicals we have in our body. So, by ensuring that IV fluids match blood plasma tonicity, doctors can safely give these fluids to patients without causing any harm.
5. Pickling
Pickling is a process where fruits or vegetables are preserved in a solution of vinegar and salt. The high salt concentration in the pickling solution creates a hypertonic environment that draws out moisture from the produce, which helps preserve it and prevent spoilage.
6. Dehydration
Dehydration happens if you’re not drinking enough liquids or losing too much water through sweating, urinating, or other bodily functions. During dehydration, the water balance in your body gets messed up, and things become more concentrated than they should be. This can cause symptoms such as thirst, dry mouth, and feeling tired, weak, and dizzy.
7. Edema
Edema is a medical condition where too much fluid builds up in your body’s tissues. This can cause swelling and discomfort in the affected area. A common cause is an imbalance between two types of pressure in your blood vessels: osmotic pressure and hydrostatic pressure. These pressures help regulate how much fluid stays inside your blood vessels versus how much leaks into surrounding tissues. When there’s an imbalance between them, more fluid may leak, leading to edema.
8. Cosmetic Products
The tonicity of cosmetic products like lotions, serums, and face masks can impact their effectiveness by affecting how well they penetrate skin cells or hair follicles. Careful formulation with appropriate tonicity levels ensures optimal absorption without causing irritation or adverse reactions.