Every football team that plays in competition always has substitute players.
That’s an extra number of players who can be quickly called in to replace another player if he gets hurt.
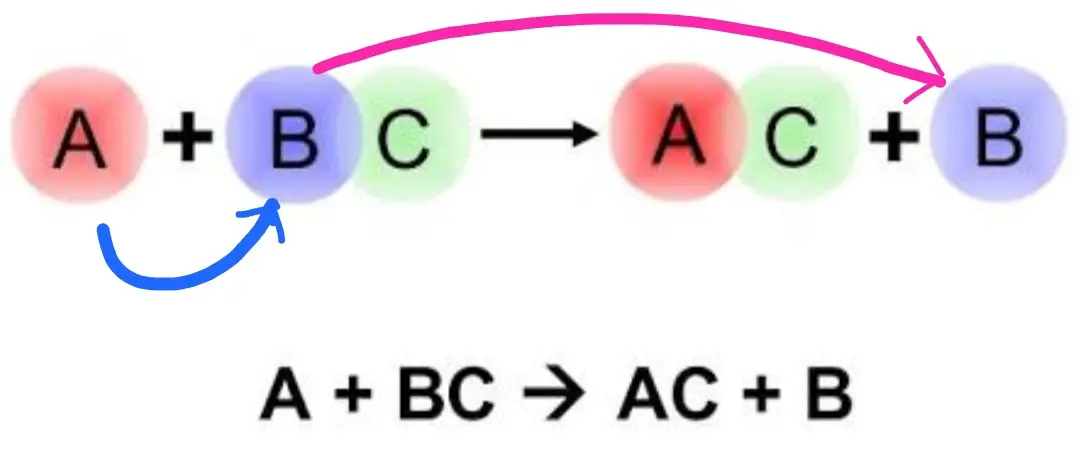
The world of chemistry also has substitutes. It happens in types of reactions called single displacement reactions.
This is a reaction where an element replaces another element in a compound during a reaction.
The starting materials in the reaction are an element and a compound that react together to produce another element and another compound.
Both of the elements in the reaction are pure elements and also both compounds are aqueous.
The elements replacing each other can be metals or nonmetals.
Single displacement reaction takes the following general form:
AY (aq) + C ⇾CY (aq) + B
As you can see, A is replaced by C in the compound AY to form CY. Also, both compounds AY and CY are aqueous which means the reactant element A began as ions and ended as an element.
Also, the reactant element C starts as an element but ends as ions in the aqueous compound CY.
Single replacements reactions are only possible if the reactant element in the reaction is more reactive than the element in the reactant compound.
Therefore, you can predict if the reaction will take place by comparing the reactivity of the elements.
Example:
F₂ + 2NaBr ⇾2NaF + Br₂
In the above reaction, bromine is replaced by fluorine. We can predict that since fluorine is more reactive than bromine, the reaction will take place and the fluorine will replace the bromine.
It also means this reaction is irreversible since bromine is a less reactive element than fluorine.
NaF + Br₂ (this reaction will not take place, so nothing happens)
Energy in single displacement reactions
Single replacement reactions are usually exothermic.
In an exothermic reaction, energy is produced—usually in the form of heat or light.
This differs from endothermic reactions where energy is absorbed or used up.
Here are 5 single displacement reaction examples in everyday life:
1. Correction of Statue of Liberty discoloration

Do ever wonder how the Stature of liberty is able to maintain its shiny copper gleam since its erection in 1889?
The external part of the Stature of Liberty is copper and the inner side is iron support.
After prolonged exposure to air, the copper patina loses its color and becomes bluish green.
These are copper compounds like copper carbonate, which are also known as verdigris.
To restore its color, a single replacement reaction between the iron support and verdigris turns the patina back to copper.
2. Extracting iron

In the steel industry, one of the processes that are performed is to purify iron from its oxides.
This is done using a carbon form called coke which is reacted with iron oxide.
2Fe₂O₃(s) + 3C(s) → 4Fe(l) + 3CO₂(g)
In this reaction, carbon displaces iron from the iron compound (also called iron (III) oxide. This is because carbon is higher in the reactivity series than iron.
3. Removing silver tarnish

Silver items such as jewelry can develop black smudges which are tarnish. This is because the silver undergoes a chemical reaction when it is exposed to hydrogen sulfide.
This produces silver sulfide which causes the silver to lose its luster. The silver color can be restored through a displacement reaction.
3Ag₂S + 2Al → 6Ag + Al₂S₃
Since aluminum is more reactive than silver, it displaces it in the compound.
4. Extracting halogens

There is a high demand worldwide for halogen gas, bromine, with almost annual 556,000 metric tonnes supplied.
Most of this comes from reserves in just 3 regions in 3 countries: Shandong province in China, the Dead Sea Waters in Israel, and Arkansas in the USA.
But how is it extracted? It is done by displacement reactions in which bromine is displaced by chlorine. The ionic equation is:
2Br- + Cl₂ → 2 Cl- + Br₂
5. Zinc extraction
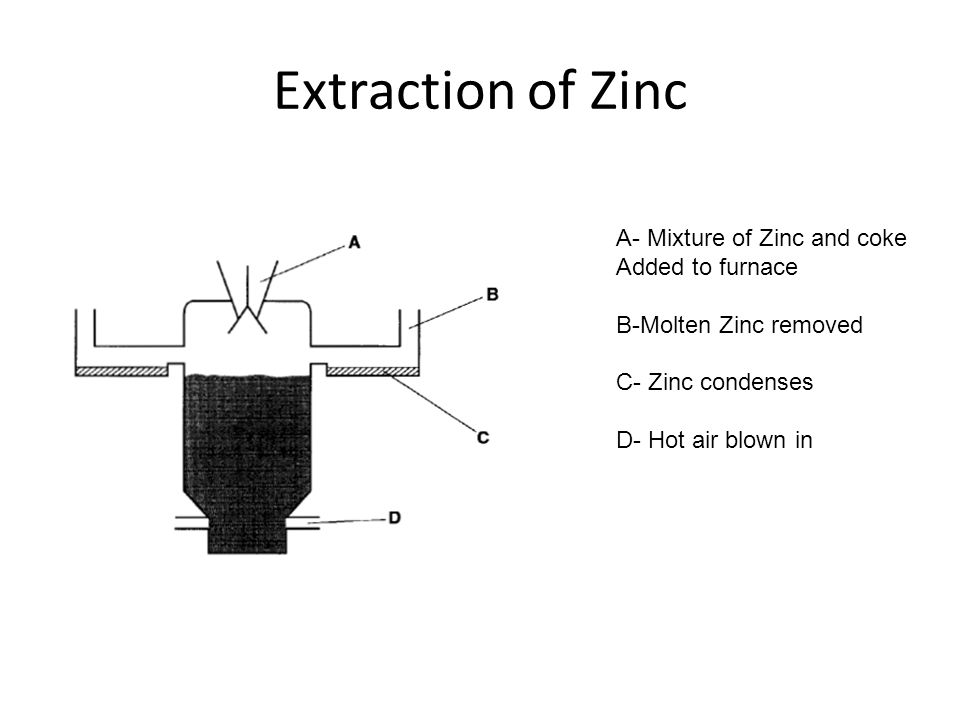
Zinc usually exists as zinc blende or zinc sulfide that is usually in crystalline form.
Extracting the zinc requires several steps including the last step of smelting.
During smelting, it is mixed with coke, a form of carbon.
The zinc oxide can be oxidized to get zinc. This is a displacement reaction since carbon displaces zinc from its oxide.
ZnO + C → Zn + CO
The zinc is replaced by carbon because the carbon is more reactive than the zinc metal in the reactivity series.
Conclusion
Chemical elements, just like people can get into fierce fights.
In some of these, a reigning champ can be overthrown from his citadel.
And similar to them, there can be only one winner and one loser in a displacement reaction.