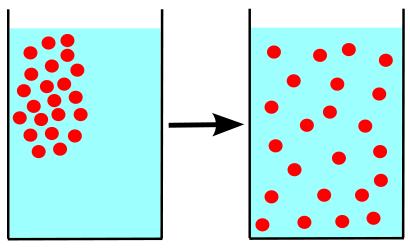
Concentration gradient is a term commonly used in biology and physical chemistry to describe the difference in concentration of a solute between two regions. This difference can occur in cells, tissues, organs, or even solutions outside the body.
Concentration Gradient Principle
The concentration gradient is based on the principle of diffusion. Diffusion is the natural tendency of a substance to spread from a region of higher concentration to a region of lower concentration. This occurs until the concentration levels are equal in both regions, known as equilibrium.
Various factors, such as temperature, pressure, and concentration, can influence the concentration gradient. For example, a higher temperature will increase the diffusion rate, whereas a higher concentration difference will result in a faster diffusion rate.
Examples of Concentration Gradient in Real Life
Concentration gradients are essential for many biological processes, such as osmosis, endocytosis, and exocytosis, and are found in various situations in our daily lives. Below are some examples.
1. Hormone Regulation
Hormones help regulate and control different functions in our bodies. They travel through our bloodstream from where there are higher amounts to the places where they are in smaller quantities. This helps them regulate and control bodily functions like growth, metabolism, and reproduction.
2. Breathing
Breathing aids in inhaling and exhaling air. It’s the process through which we take in oxygen, which is essential for our bodies to survive, and release carbon dioxide, which is a waste product.
When we breathe, oxygen moves into our lungs because there’s more outside than inside. This difference in concentration creates a concentration gradient. At the same time, carbon dioxide that has built up in our body moves out of our lungs and into the air for the same reason: there’s less of it outside than inside. Breathing helps us balance these two gases by moving them back and forth across this concentration gradient.
3. Kidney Function
The kidneys help filter out waste products from our blood. They create areas of high and low concentrations to move substances around. The kidneys also reabsorb useful substances like glucose from the filtered blood while concentrating waste products into urine. The kidneys act as a filter for our blood, removing stuff we don’t need and keeping the important things in our bodies.
4. Immune Response
The immune response is how our body fights off infections and diseases. White blood cells act as soldiers summoned to battle by migrating to where they are needed to defend against the enemy. They move towards areas with more germs or foreign substances in the body. Once they reach these areas, they work to fight off the germs and protect us from getting sick.
5. Diffusion of Perfume
Have you ever noticed how the smell of perfume or cologne spreads throughout a room after someone sprays it in one corner? This happens because of diffusion. So, when you spray perfume in one corner of a room, the particles from the spray start moving outwards towards areas with fewer particles until there is even distribution throughout the room. This process happens because there is a concentration gradient, or difference in concentration, between the sprayed area and other room parts.
Related Posts:
10 Facilitated Diffusion Examples in Real Life
6 Reverse Osmosis Examples in Everyday Life
6. Digestion of Food
When we eat food, our body needs to break it down into smaller particles so that our cells can absorb and use the nutrients. During digestion, the nutrients from food move from an area where there are many of them in our digestive tract to an area where there are fewer in our bloodstream. This means that the nutrients get absorbed through the walls of our intestines and into our blood vessels so they can be transported throughout the body to provide energy and nourishment for all our organs and tissues.
7. Global Warming
Global warming causes Earth’s temperature to increase due to certain gases in the atmosphere trapping heat. One of these gases is carbon dioxide, released by human activities such as burning fossil fuels and deforestation. It moves from an area with a high atmospheric concentration to an area with a low concentration in the ocean.
8. Rusting of Iron
Rusting happens when oxygen, which is in high concentration in the air, moves to the surface of iron with low oxygen concentration. The oxygen reacts with the iron on the surface and creates rust. This reaction can weaken the structure of iron objects over time if not properly maintained or protected from exposure to air and water.
9. Blood Clotting
Blood clotting happens when platelets, tiny cells in our blood, move toward areas with high fibrinogen levels. Fibrinogen is a protein that plays a vital role in forming blood clots. When platelets reach these areas with lots of fibrinogen, they stick together and form a clot to prevent further bleeding.
10. Kidney Dialysis
Kidney dialysis helps remove waste products from the blood of patients with kidney problems by using dialysis fluid, which goes into the patient’s body through a machine. This fluid contains chemicals that help draw out waste products from the patient’s bloodstream. During the procedure, waste products move from an area of high concentration in the patient’s blood to a region of low concentration in the dialysis fluid. This happens because molecules naturally move from areas where they are more concentrated to areas where they are less concentrated until there is equilibrium or balance between both sides.
You may also want to check:
8 Passive Transport Examples in Real Life
10 Examples of Effusion in Real Life
11. Water Filtration
The process of filtration involves passing the unfiltered water through a filter membrane. The filter membrane allows only clean water molecules to pass through while trapping the impurities on its surface. This happens because the impurities move from an area of high concentration in unfiltered water to a region of low concentration in filtered water through the filtration membrane.